ИНСТИТУТ ЦИТОЛОГИИ РОССИЙСКОЙ АКАДЕМИИ НАУК
| Лаборатория молекулярной биологи стволовых клеток |
(English version only)
Alexey TOMILINPh.D., Sci.D.,Corresponding Member of the Russian Academy of Sciences, Head of the Laboratory
Institute of Cytology
E-mail: a.tomilin@incras.ru
|

|
|
Undergraduate Studies, School of Physics, Dept. Biophysics, Polytechnical State Institute, Leningrad, USSR/Russia. 1993-1997
1998-2002
2002-2006
2007-now
|
| The laboratory, established in the Institute of Cytology in 2006, has broad interests in cell and molecular biology with a primary focus on pluripotent stem cells, such as embryonic stem (ES) and induced pluripotent stem (iPS) cells. Study of the molecular mechanisms of stem cell maintenance and development is our primary emphasis, however, we are increasingly working on developing applications of pluripotent stem cells in clinics. The most recent and exciting current topics are as follows. |
|
Mechanisms of transcriptional regulation of Oct4 gene
Oct4 gene is a central cellular player involved in the maintenance of cellular pluripotency, as well as the establishment of pluripotent cell state during in vitro reprogramming. Considering the importance of this function, a highly relevant pursuit is to dissect molecular mechanisms controlling the expression of Oct4 gene itself. We have recently identified novel factors binding and controlling Oct4 expression via the well-known distal enhancer previously shown to be essential and sufficient for Oct4 expression in pluripotent and germ cells. Studies unraveling biological role molecular details and of this regulation are currently under way. Besides fundamental aspects, the obtained knowledge may provide an important tool, for example, to improve the efficiency of iPS cell generation. |
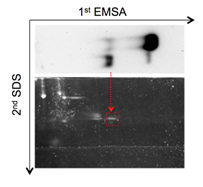
|
|
Oct4 functions in stem cells
It is becoming increasingly clear that Oct4 functions go beyond the control of identity of pluripotent and germ cells. A recent collaborative study has provided the first genetic evidence that Oct4 plays a role in some somatic cells and tissues that undergo stress during pathogenesis and tissue responses to injury (manuscript in preparation). Molecular mechanism of Oct4 induction and target genes under these stress conditions are in focus of our research. |
|
Structure of eukaryotic chromatin
|
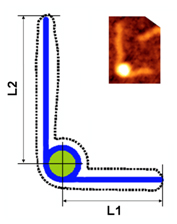
|
|
Human artificial chromosomes (HACs) for ES/iPS cell-based regenerative medicine and gene therapies
Human artificial chromosomes (HACs) are a powerful DNA vector system developed recently to introduce large chromosomal fragments, genes and regulatory elements into cultured mammalian cells without affecting the host genome. |
| This approach is devoid of known problems of viral or other vector tools such as insertional mutagenesis and unstable expression. However the delivery of HACs directly into cells of living organism is feasible so far only via cultured cells that are first to be targeted with HACs, and then incorporated into desired tissues and organs. Pluripotent stem cells such as embryo-derived embryonic stem (ES) cells and autologous induced pluripotent stem (iPS) cells seem to be an ideal choice for HAC delivery via tissue-replacement because they possess the capacity for unlimited self-renewal ex vivo and can differentiate into virtually any cell type of the organism both in vivo and in vitro (reviewed in Kouprina et al., 2014, Expert Opin. Drug Del. 11(4): 1-19). As initial step of the approach, we have recently generated and functionally evaluated mouse ES cells carrying alphoidtetO-HAC, the newest generation of in vitro assembled HACs, was transferred into mouse ES cells. |
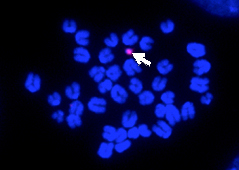
|
| Autonomous maintenance of this HAC was checked via FISH analysis of chromosome spreads. Our data suggest that alphoidtetO-HACs can be stably maintained and expressed in pluripotent and derived thereof differentiated cells without any detrimental effects on developmental processes (Liskovykh et al., 2015, Cell Cycle, in press). The result thus serves as a paradigm for the development of further HAC-based approaches to tackling a broad range of hereditary recessive diseases in human, which is currently being actively pursued in our lab using different mouse models. |
Main Group
 Dr. Sergey SINENKO
Dr. Sergey SINENKO
Staff Scientist sinenkos.a@gmail.com |
 Dr. Igor NAZAROV
Dr. Igor NAZAROV
Staff Scientist i_naz@mail.ru |
 Dr. Tatyana STARKOVA
Dr. Tatyana STARKOVA
Staff Scientist t.starkova@incras.ru |
 Sergey PONOMARTSEV
Sergey PONOMARTSEV
PhD student s.ponomartsev@incras.com |
 Evgeny BAKHMET
Evgeny BAKHMET
Ph.D. student e.bakhmet@incras.com |
 Andrey KUZMIN
Andrey KUZMIN
PhD student a.kuzmin@incras.ru |
 Alexander KHUDYAKOV
Alexander KHUDYAKOV
PhD student hudiakov.aa@gmail.com |
 Elena SKVORTSOVA
Elena SKVORTSOVA
Research Assistant e.skvortsova@incras.ru |
 Natalia KHRAMOVA
Natalia KHRAMOVA
Technitian nataliyaspb2011@yandex.ru |

Elena KUZMINA
|

Ekaterina SYTNIK
| 
Anastasia PASHUTOVA
|
|
|
|
Lab Alumni
Alexandra Kondrashkina, currently working for a company, alex_sandra2502@mail.ru;
|
COLLABORATORS
|
Dr. Vladimir Larionov and Dr. Natalia Kouprina, National Cancer Institute, NIH, Bethesda, USA;
Prof. Gary K. Owens and Dr. Olga Cherepanova, Virginia University, USA; Prof. Colyn Crane-Robinson, University of Portsmouth, UK; Prof. Michael Bader and Dr. Natalia Alenina, Max-Delbruck Center for Biomedicine, Berlin, Germany; Dr. Ulricke Seifert, Magdeburg Uiversity, Germany |
PUBLICATIONS BY LAB MEMBERS
(starting from the most recent ones; lab members are highlighted in bold)
|
2010-present
Cherepanova OA , Gomez D, Shankman LS, Swiatlowska P, Williams O, Sarmento OF, Alencar GF, Hess DL, Bevard MH, Greene ES, Murgai M, Turner SD, Geng YJ, Bekiranov S,
Connelly JJ, Tomilin A, Owens GK (2016) Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective.
Nat Medicine, published online 16 May 2016; doi:10.1038/nm.4109
Kostina AS, Uspensky VЕ, Irtyuga OB, Ignatieva EV, Freylikhman O, Gavriliuk ND, Moiseeva OM, Zhuk S, Tomilin A, Kostareva АА, Malashicheva AB (2016)
Notch-dependent EMT is attenuated in patients with aortic aneurysm and bicuspid aortic valve. Biochim Biophys Acta. 2016 Feb 10. pii: S0925-4439(16)30032-1.
doi: 10.1016/j.bbadis.2016.02.006. [Epub ahead of print]
Nazarov I, Chekliarova I, Rychkov G, Ilatovskiy AV, Crane-Robinson C, Tomilin A. (2016) AFM studies in diverse ionic environments of nucleosomes reconstituted
on the 601 positioning sequence. Biochimie 121: 5-12.
Liskovykh M, Ponomartsev S, Popova E, Bader M, Kouprina N, Larionov V, Alenina N, Tomilin A. (2015) Stable maintenance of de novo assembled human artificial
chromosomes in embryonic stem cells and their differentiated progeny in mice. Cell Cycle 4(8):1268-73.
Kulichkova VA, Artamonova TO, Zaykova JJ, Ermolaeva JB, Khodorkovskii MA, Barlev NA, Tomilin A, Tsimokha AS (2014) Simultaneous EGFP and Tag Labeling of
the b7 Subunit for Live Imaging and Affinity Purification of Functional Human Proteasomes. Mol Biotechnol. Aug 28. [Epub ahead of print].
Bajenova O, Chaika N, Tolkunova E, Davydov-Sinitsyn A, Gapon S, Thomas P, O'Brien S. (2014) Carcinoembryonic antigen promotes colorectal cancer progression
by targeting adherens junction complexes. Exp Cell Res. Epub 2014 Apr 12.
Kouprina N, Tomilin A, Masumoto H, Earnshaw WC, Larionov V. (2014) HAC-based gene delivery vectors for gene function studies, gene therapy and pharmacology.
Expert Opin. Drug Del. 11 (4): 1-19.
Yang CS, Sinenko SA, Thomenius MJ, Robeson AC, Freel CD, Horn SR, Kornbluth S. The deubiquitinating enzyme DUBAI stabilizes DIAP1 to suppress
Drosophila apoptosis. Cell Death Differ. 2014 Apr;21(4):604-11. doi: 10.1038/cdd.2013.184. Epub 2013 Dec 20.
DeVeale B, Brokhman I, Mohseni P, Babak T, Yoon C, Lin A, Onishi K, Tomilin A, Pevny L, Zandstra PW, Nagy A, van der Kooy D (2013) Oct4 is required for e7.5 for
proliferation in the primitive streak. PLoS Genet. 9(11): e1003957.
Wu G, Han D, Gong Y, Sebastiano V, Gentile L, Singhal N, Adachi K, Fischedick G, Ortmeier C, Sinn M, Radstaak M, Tomilin A, Schöler HR. (2013) Establishment of
totipotency does not depend on Oct4. Nat Cell Biol. 15(9): 1089-97.
Lee N.C.O, K. A., Lee H.S, Tolkunova E.N., Liskovykh М.A., Masumoto H., Earnshaw W.C., Tomilin A., Larionov V., Kouprina N. (2013) Protecting a
transgene expression from the HAC-based vector by different chromatin insulators. Cell. Mol. Life Sci. 70(19): 3723-37.
Nazarov I, Krasnoborova V, Mitenberg A, Chikhirzhina E, Davidov-Sinitsin A, Liskovykh M, and Tomilin A (2014) Transcription Regulation of
Oct4 (Pou5F1) Gene by Its Distal Enhancer. Cell and Tissue Biol 8(1): 27-32.
Davydov-Sinitsyn A, O. V. Bazhenova, M. A. Liskovykh, L. L. Chechik, S. V. Ponomartsev S, Tomilin A, Tolkunova E. (2013) In vitro Derivation and Characterization
of a Colorectal Cancer Stem Cell Subpopulation. Cell and Tissue Biol 7(4): 320-325.
Davydov-Sinitsyn A, Bajenova O, Liskovykh M, Ponomartsev S, Chechik L, Tomilin A, Tolkunova E. (2012) Comparative Analysis of Colorectal Carcinoma Cell Lines
That Differ in Metastatic Potential. Cell and Tissue Biol 7(5): 407-412.
Liskovykh M., Chuykin I., Ranjan A., Safina D., Popova E., Tolkunova E., Mosienko V., Minina J., Zhdanova N., Mullins J.J., Bader B.,
Alenina N., Tomilin A. (2011) Derivation, characterization, and stable transfection of induced pluripotent stem cells from Fischer344 rats. PLoS ONE
11 (6): e27345.
Liskovykh M, Davydov-Sinitcyn A, Marilovtceva E, Tomilin A, Tolkunova E. (2011) Interaction between the CDX2 Transcription Factor and DDX5 Protein.
Cell and Tissue Biol. 6(1): 20-25.
Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. (2011) Interaction between differentiating cell- and
niche-derived signals in hematopoietic progenitor maintenance. Cell 147(7): 1589-600.
Sinenko SA, Shim J, Banerjee U. (2011) Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila.
EMBO Rep. 13(1):83-9
Sinenko SA, Hung T, Moroz T, Tran QM, Sidhu S, Cheney MD, Speck NA, Banerjee U (2010) Genetic manipulation of AML1-ETO-induced expansion of hematopoietic
precursors in a Drosophila model. Blood 116(22): 4612-20.U.
Kuckenberg P., Buhl S., Woynecki T., van Fürden B., Tolkunova E., Seiffe F., Moser M., Tomilin A., Winterhager E., Schorle H. (2010) The
transcription factor TCFAP2C/AP-2gamma cooperates with CDX2 to maintain trophectoderm formation. Mol. Cell. Biol. 30: 3310-3320.
[ download ]
Tomilin A., Tolkunova E., Liskovykh M. (2009) A method of stem cell application for tissue-replacement therapies. 2009 Russian Federation patent application
(2009143025).
Saxe J., Tomilin А., Schöler H.R., Plath K., Huang J. (2009) Post-Translational Regulation of Oct4 Transcriptional Activity. PLOS One 4: 1-9.
[ download + supplement ]
Tolkunova E., Malashicheva A., Chikhirzhina E., Kostyleva E., Zeng W., Luo J., Dobrinski I., Hierholzer A., Kemler R., and Tomilin A. (2009)
E-Cadherin as a Novel Surface Marker of Spermatogonial Stem Cells. Cell and Tissue Biol. 3: 103-109.
[ download ]
Popov B., Petrov N., Mikhailov V., Tomilin A., Alekseenko L., Grinchuk T., and Zaichik A. (2009) Spontaneous Transformation and Immortalization of
Mesenchymal Stem Cells in vitro. Cell and Tissue Biol. 3: 110-115.
Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. (2009) Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila.
Dev Cell 16(5): 756-63.
Tolkunova A., Malashicheva A., Parfenov V.N., Sustmann C., Grosschedl R., Tomilin A. (2007) PIAS proteins as repressors of Oct4 function.
J. Mol. Biol. 374: 1200-1212.
[ download + supplement ]
Lengner C.J., Camargo F.D., Hochedlinger K., Welstead G.G., Zaidi S., Gokhale S., Schöler H.R., Tomilin A., Jaenisch R. (2007) Oct4 expression is not
required for mouse somatic stem cell self-renewal. Cell Stem Cell 1: 403-415.
[ download + supplement ]
Malashicheva A., Kanzler B., Tolkunova E., Trono D., Tomilin A. (2007) Lentivirus as a tool for lineage-specific gene manipulations.
Genesis 45, 456-459.
[ download + supplement ]
Tolkunova E., Cavaleri F., Eckardt S., Reinbold R., Christenson L.K., Schöler H.R. , Tomilin A. (2006) The Caudal-Related Protein Cdx2
Promotes Trophoblast Differentiation of Mouse ES Cells. Stem Cells 24: 139-144.
[ download + supplement ]
Kehler J, Tolkunova E., Koschorz B., Pesce M., Gentile L., Boiani M, Lomeli H, Nagy A, McLaughlin K.J., Schöler H.R., Tomilin A. (2004)
Oct4 is required for primordial germ cell survival. EMBO Rep. 5: 1078-83.
[ download + supplement ]
Lins K., Reményi A., Tomilin A., Massa S., Wilmanns M., Matthias P. and Schöler H.R. (2003) OBF1 enhances transcriptional potential of Oct1.
EMBO J. 22: 2188-2198.
[ download ]
Seralini G.E., Tomilin A., Auvray P., Nativelle-Serpentini C., Sourdaine P., Moslemi S. (2003) Molecular characterization and expression of equine testicular
cytochrome P450 aromatase. Biochim Biophys Acta. 1625: 229-238.
[ download ]
Kobayashi M., Fujioka M., Tolkunova E., D. Deka, M. Abu-Shaar, R. S. Mann, and J.B. Jaynes. (2003) Engrailed cooperates with extradenticle and homothorax
to repress target genes in Drosophila. Development 130: 741-51.
[ download ]
Remenyi A, Tomilin A, Schöler HR, Wilmanns M. (2002) Differential activity by DNA-induced quarternary structures of POU transcription factors.
Biochem Pharmacol. 64: 979-84.
[ download ]
Perez-Martinez X, Funes S, Tolkunova E, Davidson E, King MP, Gonzalez-Halphen D. (2002) Structure of nuclear-localized cox3 genes in Chlamydomonas
reinhardtii and in its colorless close relative Polytomella sp. Curr Genet. 40: 399-404.
[ download ]
*Remenyi A., *Tomilin A., Pohl E., Lins K., Philippsen A., Reinbold R., Scholer H.R., and Wilmanns M. (2001) Differential transcriptional activity of dimeric
Oct-1 by DNA-motif induced domain swapping. Mol. Cell 8: 569-580.
[ download ]
Perez-Martinez X., Antaramian A., Vazquez-Acevedo M, Funes S, Tolkunova E, d'Alayer J, Claros MG, Davidson E, King MP, Gonzalez-Halphen D. (2001)
Sububunit II of cytochrome c oxidase in Chlamydomonad algae is a heterodimer encoded by two independent nuclear genes. J Biol Chem 276: 11302-9.
[ download ]
#Tomilin A., #Remenyi A., Lins K., Bak H., Leidel S., Vriend G., Wilmanns M., Schöler H.R. (2000) Synergism with the coactivator OBF-1 (OCA-B, BOB-1)
is mediated by a specific POU dimer configuration. Cell 103: 853-864.
[ download ]
Perez-Martinez X., Vazquez-Acevedo M., Tolkunova E., Funes S., Claros MG, Davidson E., King D., Gonzales-Halphen D. (2000) Unusual location of
mitochondrial gene. Subunit III of cytochtome C oxidase is encoded in the nucleus of C. algae. J Biol Chem. 275: 30144-30152.
[ download ]
Tolkunova E., Park H., Xia J., King MP., Davidson E. (2000) The human lysyl-tRNA synthetase gene encodes both the cytoplasmic and mitochondrial enzymes
by means of an unusual alternative splicing of the primary transcript. J Biol Chem 275: 35063-35069.
[ download ]
Kropotov A., Sedova V., Ivanov V., Sazeeva N., Tomilin A., Krutilina R., Oei S.L., Griesenbeck J., Buchlow G., Tomilin N. (1999) A novel human DNA-binding
protein with sequence similarity to a subfamily of redox proteins which is able to repress RNA-polymerase-III-driven transcription of the Alu-family retroposons in vitro.
Eur. J. Biochem. 260: 336-346.
Tomilin A., Vorob'ev V., Drosdowsky M., Seralini G.-E. (1998) Oct3/4-associating proteins from embryonal carcinoma and spermatogenic cells of mouse.
Mol. Biol. Rep. 25: 103-109.
Tolkunova E., Fujioka M., Kobayashi M., Deka D., Jaynes J. (1998) Two distinct types of repression domain in engrailed: one interacts with groucho co-repressor
and is preferentially active in integrated target genes. Mol. Cell. Biol. 18 (5): 2804-2814.
download.
Belokopytova I.A., Kostyleva E.I., Tomilin A., Vorob'ev V.I. (1993) Human male infertility may be due to a decrease of the protamine P2 content in sperm
chromatin. Mol. Reprod. Dev. 34: 53-57.
|
FINANCIAL SUPPORT
|
Current financial support:
• Russian Science Foundation grant 14-15-00718; • Russian Science Foundation grant 14-50-00068; Past financial support:
•
Russian Foundation for Basic Research (14-04-31556_mol_a) to Liskovykh M./Ponomartsev S. (2014-2015);
•
Russian Foundation for Basic Research (13-02-00923a) (2013-2015);
•
Grant program "Molecular and Cellular Biology" by the Presidium of the Russian Academy of Science (2006-2012);
•
State contracts with the Ministry of Science and Education (02.512.11.2253, 16.512.11.2085, 16.512.11.2242, 2008-2012);
•
Russian Foundation for Basic Research (RFBR) - Helmholtz Society (Germany) joint grant (07-04-92281/HRJRG-024, 2009-2011);
•
Russian Foundation for Basic Research (RFBR, 07-04-01154a, 2007-2008);
|
-
⇑
Beginning
| | Главная | Научные подразделения | |