|
The main direction of studies
At the present the main direction of studies of the Group is associated with morpho-functional analysis of bases of transepithelial (transmembranous, paracellular,
transcellular, intercellular) transport of various substances.
The goal of work of the Group is to study morphological correlates of transepithelial transport of water and several sugars across epithelia that have different water
and ion permeability: permeable epithelium of rat small intestine and their model - culture cells Caco2, tight epithelium of frog urinary bladder and their model - culture
cells MDCK. In studies of the Group the following problems are analyzed: submicroscopic organization of cell plasma membrane and specialized intercellular contacts
of various types; participation of intercellular contacts in intercellular communication and in paracellular transport of substances across epithelium of the rat small intestine
and frog urinary bladder; insertion of water channel proteins, aquaporins, into the plasma membrane of granular cells of the frog urinary bladder epithelium during stimulation
of water transport; participation of Golgi apparatus in secretion of aquaporins and some proteins of the membrane and polar tube of protozoa - microsporidia; the
three-dimensional reconstruction of Golgi apparatus of microsporidia; osmoregulating role of giant vacuoles and their origin in cells during stimulation of large water flows
across the tight epithelium of frog urinary bladder; role of actin filaments and microtubules in transcellular transport of substances across epithelium; peculiarities of dynamics
of non-centrosomal microtubules in osmoregulating epithelium.
In the work of the Group, a large spectrum of current morphological methods is used: standard methods of electron and light microscopy; cryomethods of electron
microscopy (freeze-fracture, freeze-substitution, frozen ultrathin sections); immune electron microscopy; immune confocal microscopy; X-ray-structural and X-ray-spectral
analysis. Researchers of the Group have made an essential contribution to the modernization and development of some of the above mentioned methods.
Illustration of methods used at the Group
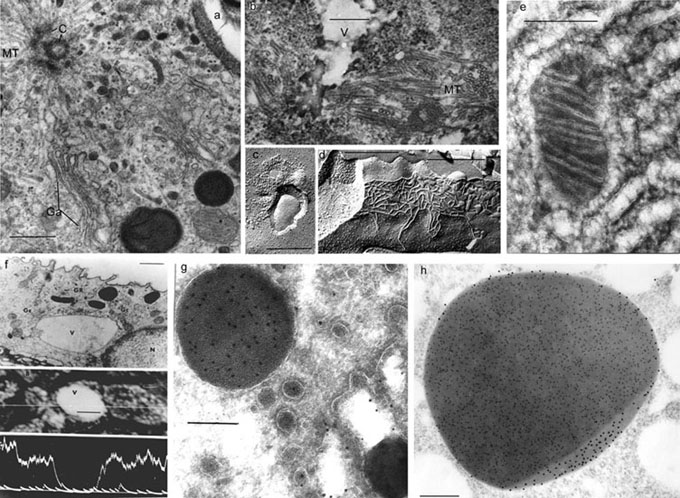
Electron Microscopy
ŗ. Routine fixation (glutar-osmium), an area of granular cell of the frog urinary bladder: centriole (—), microtubules (MT), Golgi apparatus (Gŗ),
scale bar - 0.5 μm.
b. Freeze-substitution, a giant vacuole (V) of granular cell, surrounded by microtubules (MT), at water transport induced by antidiuretic
hormone (ADH), scale bar - 0.25 μm.
Ů. Freeze-fracture, insertion of AQP2 proteins into the granular cell apical membrane during stimulation of water flow by ADH, scale bar - 0.25 μm.
d. Freeze-fracture, tight junction (TJ) between granular cells of the frog urinary bladder epithelium, scale bar - 0.25 μm.
e. Freeze-drying, an area of mouse pancreas endocrine cell; membranes of smooth endoplasmic reticulum and mitochondria are presented,
scale bar - 0.25 μm.
f. X-ray microanalysis, a low potassium concentration in granular cell giant vacuoles during ADH-induced water transport is shown, scale bar - μm.
g. Immunolabeling of cryoultrathin sections, receptor-mediated vitellogenin endocytosis and its accumulation in the mosquito oocyte yolk granule,
scale bar - μm.
h. Double immunolabeling in acryl sections, mature yolk granule of mosquito oocyte: vitellogenin (gold - 10 nm), protein VCP (gold - 15 nm),
scale bar - 0.5 μm.
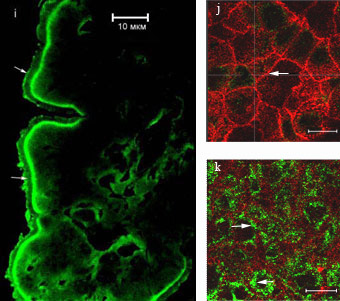
Confocal Microscopy
i. The localization of glucose transporter GLUT2 in enterocytes during absorption of high concentration glucose. Immunolabeling on frozen sections:
IAB - polyclonal antibodies against GLUT2, II AB - antibodies conjugated with Alexa-488, scale bar - 10 μm.
j-k. The distribution of GLUT2 in Caco2 cell culture after 2.5 mM glucose load, scale bar - 10 μm.
j. Horizontal optic section of cell culture at apical cell region; actin (red) is revealed at microvilli and cell boundary (arrows).
k. Horizontal optic section of cell culture at basal cell region; the label for GLUT2 (green, arrows) was mainly detected in the basal cytoplasm of the cells.
The work of the Group from 1989 to 2010 was constantly supported by grants of the Russian Foundation for Basic Research. For 6 years, the work has been
supported by international grants INTAS. The Group participated in the program of the Division of Biological Sciences of the Russian Academy of Sciences "Integrative
mechanisms of regulation of functions in organism".
Main publications
- Komissarchik Ya.Yu., Levin S.V. 1964. Distribution of phtalocyanine dye (Heliogen Blue SBL) in ultrastructures of vital dyed axons of the crab Carcinus
maenas. Nature. 204 : 203-205.
- Snigirevskaya E.S., Komissarchik Ya.Yu. 1980. Ultrastructure of specialized intercellular contacts. Tsitologiya. 22 (9) :
1011-1136.
- Komissarchik Ya.Yu., Korolev E.V., Snigirevskaya E.S. 1981. Investigation of membrane complexes of myelin and gregarine pellicle by freeze-drying and
freeze-etching methods. Acta histochem., Suppl. 23 : 275-281.
- Komissarchik Ya.Yu., Snigirevskaya E.S., Romanov V.I., Sabinin G.V. 1985. Analysis of ultrastructural
changes of apical membranes of frog urinary bladder granular cells during the ADH-stimulated osmotic water flow. Biol. Membrany. 2 (6) : 630-641.
- Snigirevskaya E.S., Komissarchik Ya.Yu. 1984. X-ray microanalysis of frog urinary bladder granular cells under conditions of enhanced water transport.
Tr. Vses. Simp. "Cryogenic methods in electron microscopy", pp. 62-64.
- Komissarchik Ya.Yu., Natochin Yu.V., Snigirevskaya E.S., Shakhmatova E.I. 1985. Specific vacuolation of frog urinary bladder
granular cell after ADH-stimulation of water transport. Gen. Physiol. Biophys. 4 : 557-572.
- Snigirevskaya E.S. 1990. Change of ultrastructure of cells of
vasopressin-sensitive epithelia during stimulation of water transport. Tsitologiya. 32 (9) : 766-794.
- Komissarchik Ya.Yu., Snigirevskaya E.S., Ugolev A.M. 1992.
Analysis of distribution of actin filaments in rat small intestine enterocytes in the process of absorption nutrients (immunoelectron microscopic study). DAN SSSR. 322 (4) :
795-797.
- Snigirevskaya E.S., Komissarchik Ya.Yu. 1993. A novel type of micrÓtubules in the frog urinary bladder epithelium stimulated by vasopressin. J. Submicrosc.
—ytol. Pathol. 25 : 389-396.
- Ugolev A.M., Komissarchik Ya.Yu., Gromova L.V., Grusdkov A.A., Snigirevskaya E.S., Brudnaya M.S. 1995. Structural and functional
analysis in glucose absorption mechanism in the rat small intestine in vivo. Gen. Physiol. Biophys. 14 : 405-417.
- Natochin Y.V., Parnova R.G., Schakhmatova E.I., Komissarchik Ya.Yu., Brudnaya M.S., Snigirevskaya E.S. 1996. AVP-independent high osmotic water
permeability of frog urinary bladder and autocoids. Pflugers Arch.
Eur. J. –hysiol. 433 : 136-145.
- Parnova R.G., Shakhmatova E. I., Plesneva S.A., Getvanova E.V., Korolev E.V., Komissarchik Ya.Yu., Natochin Yu.V. 1997. Role
of prostaglandin E2 in regulation of low and high water osmotic permeability in frog urinary bladder. Biochem. Biophys. Acta. 1356 : 160-170.
- Snigirevskaya E.S., Hays A.R., Raihkel A.S. 1997. Secretory and internalization pathways of mosquito yolk proteins. Cell Tissue Res. 290 : 129-142.
- Snigirevskaya E.S., Sappington T.W., Raikhel A.S. 1997. Internalization and recycling of vitellogenin receptor in mosquito oocytes. Cell Tissue Res. 290 : 175-183.
- Komissarchik Ya.Yu., Snigirevskaya E.S., Schakhmatova E.I., Natochin Yu.V. 1998. Ultrastructural correlates of increase in the epithelial osmotic water permeability
without antidiuretic hormone (ADH). Cell Tissue Res. 293 : 517-524.
- Snigirevskaya E.S., Komissarchik Ya.Yu. 1999. Acquporins of epithelial cell plasma membranes. Tsitologiya. 41 (10) : 864-870.
- Snigirevskaya E.S., Komissarchik Ya.Yu. 2000. Structural correlates of the trans-epithelial water transport. Int. Rev. Cytol. 198 : 203-275.
- Snigirevskaya E.S., Komissarchik Ya.Yu. 2002. Dynamics of epithelial cell microtubules. Tsitologiya. 44 (6) : 507-517.
- Komissarchik Ya.Yu., Snigirevskaya E.S. 2002. Giant vacuoles during ADH-induced transcellular bulk water flow across the epithelium of the frog urinary bladder.
Cell Biol. Internat. 26 : 373-383.
- Snigirevskaya E.S., Raikhel A.S. 2004. Receptor-mediated endocytosis of yolk proteins in insect oocytes. In: Raikhel, A.S., Sappington, T.W. (eds.)
Reproductive Biology of Invertebrates, vol. 12, Part B: Progress in Vitellogenesis, Science Publishers, Inc., Enfield, USA/Plymouth, UK, pp. 199-229.
Yu.Ya. Sokolova, E. S. Snigirevskaya, Ya. Yu. Komissarchik.
2007. Golgi apparatus in parasitic protists (review of the literature). Cell and Tissue Biology, 11 (4) : 305-327.
- G.V. Beznousenko, V.V.Dolgikh, E.V. Seliverstova, P.B.
Semenov, Yu.S. Tokarev, A. Trucco, M. Micaroni, D.Di Giandomenico, P. Auinger, I.V.Senderskiy, S.O. Skarlato, E.S. Snigirevskaya, Ya.Yu. Komissarchik, M. Pavelka,
M.A. De Metteis, A. Luini, Yu.Ya. Sokolova, A.A. Mironov. 2007. Analogs of the Golgi complex in microsporidia: structure and avesicular mechanisms of function.
J. Cell Science, 120 (7) : 1288-1298.
- Gorshkov A.N., Snigirevskaya E.S., Komissarchik Ya.Yu. 2009. Arginin-vasopressin-induced alterations in the structure of
MDCK cells. Tsitologiya. 51 (2) : 111-121.
- Gruzdkov A.A., Gromova L.V., Grefner N. M., Komissarchik Ya. Yu. 2010. Geometric peculiarities of intestinal surface and efficiency of coupling between membrane
hydrolysis and transport of nutrients // Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology.. Vol. 4. No 3. pp. 269-277.
- >Grefner N. M.. Gromova L. V., Gruzdkov A. A., Komissarchik Ya. Yu.
Comparative analysis of SGLT1 and GLUT2 transporters distribution in rat small
intestine enterocytes and Caco_2 cells during hexose Absorption//Cell and tissue biology.
2010. Vol. 4, Ļ 4. P. 354 - 361.
- Gruzdkov A., Gromova L., Grefner N., Komissarchik Y. 2012. Kinetics and mechanisms
of glucose absorption in the rat small intestine under physiological conditions. Journal of
Biophysical Chemistry, 3 (2):191-200.
- Mosevitsky M.I., Snigirevskaya E.S., and Komissarchik Ya.Yu. 2012. Immunoelectron
microscopic study of BASP1 and MARCKS location in the early and late rat
spermatids. Acta Histochem, 114 (3): 237-243.
- Vishnyakov I.E., Levitskii S.A., Manuvera V.A., Lazarev V.N., Ivanov V.A.,
Snigirevsk., Komissarchik Ya.Yu., and Borchsenius S.N. 2012. The identification and
characterization of IbpA, a novel apha-crystallin-type heat shock protein from
mycoplasma. Cell Stress Chaperons, 17 (2): 171-180.
|


