| Laboratory of Intracellular Membranes Dynamics |
Elena S. KORNILOVAPh.D., Doctor of Biological Sciences, Professorphone: +7 (812) 297-4596
The Lab was founded in 2005th on the basis of the Vesicular Traffick Group of Cell Cycle Physiology Laboratory and the Biochemistry of Vacuolar Apparatus Group of the Laboratory of Biochemical Cytology and Cytochemistry. Stuff: 8 researchers (including 2 DBS and 4 PhD). |

|

|
The main attention in the Lab is paid to studies of EGF receptor endocytosis - the process stimulated simultaneously with formation of EGF-receptor complexes at the plasma
membrane. 3 main directions of research include : (1) analysis of the role which the receptor ubiquitination plays in regulation of its lysosomal degradation; (2) the role of tubulin
cytoskeleton in coordination of EGF receptor endocytosis and signaling; (3) studies of alteration in endocytosis regulation during cell differentiation. Besides we conduct studies
aimed to evaluate the possible range of new semiconductor fluorescent nanocrystals (Quantum Dots, QDots) application in basic and applied areas of cell biology.
The work of the Laboratory is supported by grants of Russian Foundation for Basic Research, Government of Sankt-Petersburg, grants for Young Scientists, by Programs of RAS "Molecular and Cell Biology" and "Basic Research in Nanothechnolohy and Nanomaterials", by the President of Russian Federation grant for Science Schools, by Russian Agency for Science and Innovations. The members of the Lab have experience of working in the Labs of Great Britain, Switzerland, USA, Germany, Belgium , Canada and Finland. Many peptide hormones and growth factors stimulate various cellular responses mediated by their highly specific transmembrane receptors. As a rule, these receptors are coupled to intracellular tyrosine kinases or possess internal tyrosine kinase (TK) localized in cytoplasmic receptor domain. The latter case is represented by one of the widely expressed receptor - the receptor for epidermal growth factor (EGF), which regulates a spectrum of cellular reactions, from proliferation to differentiation, cell migration or survival. Soon upon EGF receptor identification (in late 1970ths) it became clear that EGF-receptor complexes enter the cell via endocytosis and are delivered to lysosomes, where the both ligand and receptor are degraded. Thus, the main role of endocytosis was suggested to attenuate the signal generated at plasma membrane. However, the last decade brings numerous data indicating independent signaling role for endosomal receptor. It was shown that internalized receptor is able to generate new signals, and so many different proteins, protein complexes and lipids are involved in organization of endocytic pathway that the degradation of EGF receptor could hardly be the main aim. So the question on the role of endocytosis of signaling receptors remais mostly open. The current studies in the Lab continue research that was conducted by the Lab members during many years. During this time we have shown that internalized receptor is not obligatory delivered to lysosomes for degradation, but may escape by entering the recycling pathway. Importantly, the ratio of these two ways changed in wide range in the cells of the same cell line. We have demonstrated that C-terminal regulatory receptor domain with major tyrosine phosphorylaton sites modulates the efficiency of the receptor sorting for degradative pathway. Studies of small GTPases Rab 5 and Rab7 and PI3P-kinases provide data in favor of maturation model of early-to-late endosome transition. Analyzing the role of ubiquitin-ligase c-Cbl, we find that the current status of the receptor ubiquitination results from dynamic balance of ubiqiutinating and deubiquitinating enzymatic activities along all the endocytic pathway. Dymanics of ubiquitination patterns' alterations allows to suggest the labile ubiquitination is that mechanism which allows to coordinate consecutive interactions of the receptor with sorting ESCRT complexes. These and other data provide the basis for understanding of EGF receptor endocytosis as extremely flexible process which is controlled not only by EGF but depends on the overall signaling microeviromental context. Taking into account new data on cross-talks of endocytotic regulating proteins and different signaling cascades it becomes obvious that such a flexibility provide a cell higher adaptability and influences the tolal output signaling responses generating by EGF in different conditions. Thus, endocytosis may play a key role as integrator of numerous signaling pathways "in time and place" inside a cell. The main project at present is the study of cross-talk of EGF receptor endocytosis and another integral cell system, namely, tubulin cytoskeleton, or microtulules (MTs). Despide the idea if interphase microtubules as of stabile railway-like system with dynamics instability of individual rails, which main role in endocytosis is to transports endosomal vesickles toward the cell center by mean of motor protein, we find that MTs after stimulation of EGF receptor endocytosis undergo essential remodeling due to changing MTs' properties. The main aim of this work is to reveal the mechanisms of found remodeling,to understand how these changes influence endocytosis and whether the endosomal receptor is involved in initiating or interrupting certain remodeling steps. We are also looking for other (EGF-independent) signals which can influence EGF receptor-containing endosome cross-talk with MTs and MT-regulating proteins.We focuses on the role of regulatory endocytic proteins and MT-stabilizing or severing proteins, as well as the role of posttranslation MT modifications. Possible involvement of endosomal acidification and local redox-status, local changes of Ca2+ level in MTs-reorganizations and endosome maturation is under investigation. 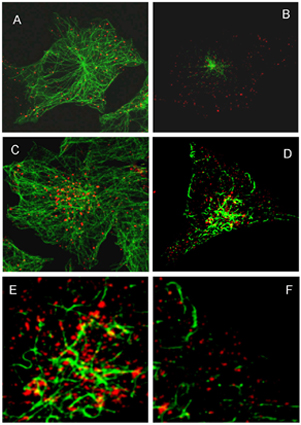
Fig1. Endosome enlargement at late stages of endocytosis occurs on highly acetylated microtubules (MTs)
The cells were fixed at 15 (A, B) and 60 (C – D) min of endocytosis and stained with primary Abs against EGF receptor and alpha-tubulin (A, C) and EGF receptor and acetylated tubulin (B, D – F) followed by staining with corresponding secondary flluorescent Abs. E and D are enlarged fragments of juxtanuclear and peripherical areas of the cell presented on image D. The images were taken with use of LEICA TCS SL. The second project conserns the study of changes in plasma membrane properties and endocytosis characteristics durinf differentiaton process. As a model
stimulated differentiation of cultured mioblasts into moitubules is used.
The third direction of our work concerns possible application of new type semiconductor fluorescent crystals of nanosize (2-9 nm), so called Quantum Dots (QDs), in basic research and for diagnostics and therapy. Their unique optical properties - high quantum yeld, photostability, size-determined emission range, big Stocks shift and others, - make them attractive markers for long-term and sinlge molecule detection of proteins of interest. However, toxic material they are made of, significant increase in size due to functionalization (which is necessary to make them soluble in biological buffers and to provide specificity) rize many questions which have to be solved before QDs' introduction in practice. Our main goal is to determine how different methods of functionalization and introducing into the cell can affect the fate of QD-labeled molecules inside the cell, how do they exit the cell and what is the general reaction of an organizm on their application. These questions are very important if the molecule labeled with QD has signaling function, such as EGF. The main issue we address in our study is to reveal whether labeling EGF with QDs could influence its signaling. This aspect is essential for development of adequate approaches for implicating QDs in diagnostics and therapy. 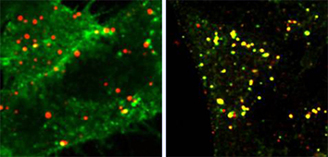
Fig. 2 QDots conjugated to EGF enter HeLa cells via EGF receptor-mediated endocytosis
Left image: fixed cells were not treated with Triton X-100, as a result the Abs have stained only surface receptor while EGF was localized mostly in endosomes. Right image: upon permeabilization receptor antibody co-localize with DQs-labeled EGF. Thus, EGF labeling with QDots does not interfere the specofic receptor-mediated endocytosis of EGF-receptor complexes.
Salova A.V., Leontieva E.A., Mozhenok T.P., Kornilova E.S., Krolenko S.A., Beliaeva T.N. 2011. Alterations in localization of cell vesicular apparatus components during differentiation of cultured myoblasts into myotubules. Tsitologiya (Rus). in press Zlobina MV, Kharchenko MV, Latkin DS, Kornilova ES.. 2010. Acetylation of microtubules during endocytosis of epidermal growth factor receptor (c-ErbB1) in interphase HeLa cells.Tsitologiya. (Rus). 52(6):466-76. Kirill A. Kondratov, Alexander L. Chernorudskiy, Alina P. Amosova and Elena S. Kornilova. 2010. Termination of tyrphostin AG1478 application results in different recovery of EGF receptor tyrosine residues 1045 and 1173 phosphorylation in A431 cells. Cell Biol Int., 34 (1):81-87 Beliaeva TN, Krolenko SA, Leont'eva EA, Mozhenok TP, Salova AV, Faddeeva MD. 2009. AO distribution and fluorescence spectra in myoblasts and single muscle fibres. Tsitologiya. (Rus). 51(2):103-10 Kondratov KA, Chernorudskii AL, Amosova AP, Kornilova ES.2009. Analysis of tyrphostin AG1478 effect on behavior of internalized EGF receptor at different stages of endocytosis. Tsitologiya. (Rus). 51(6):520-5. Kondratov KA, Melikova MS, Chernorudskii AL, Kornilova ES. 2009. Ubiquitination of EGF receptors with C-terminal domain deletion and point mutations during endocytosis.Tsitologiya. (Rus). 51(7):617-23. Beliaeva TN, Salova AV, Leont'eva EA, Mozhenok TP, Kornilova ES, Krolenko SA. 2009. Untargeted quantum dots in confocal microscopy of living cells. Tsitologiya. (Rus). 51(10):830-7. Melikova Maria S, Kondratov Kirill A, Kornilova Elena S. 2006. Two different stages of epidermal growth factor (EGF) receptor endocytosis are sensitive to free ubiquitin depletion produced by proteasome inhibitor MG132. Cell Biol Int.30: 31-43. Kharchenko M. V., Aksyonov A. A., Melikova M. M., E.S. Kornilova . 2007. Epidermal growth factor (EGF) receptor endocytosis is accompanied by reorganization of microtubule system in HeLa cells. Cell Biol. Int.. 31 : 349-359. Krolenko SA, Adamian SIa, Beliaeva TN, Mozhenok TP, Salova AV. 2007. Confocal-microscopic study of skeletal muscle fibre membrane organelles during Zenker's (spreading) necrosis .Tsitologiya (Rus). 49(2):107-14 Zheleznova NN, Melikova MS, Kharchenko MV, Nikol'skii NN, Kornilova ES. 2003. The role of phosphatidylinositol-3-kinases P85/P110 and hVPS34 in endocytosis of EGF-receptor complexes.Tsitologiya Rus).;45(6):574-81. T. Belyaeva, E. Leontieva, A. Shpakov, T. Mozhenok, M. Faddejeva. 2003. Sensitivity of lysosomal enzymes to the plant alkaloid sanguinarine: comparison with other SH-specific agents. Cell Biol. Int. 27: 887-895 Krolenko SA, Adamian SIa, Beliaeva TN, Mozhenok TP. . 2003. Localization of acid organelles in frog skeletal muscle fibers. Tsitologiya;45(7):714-21. Melikova MS, Filatova MM, Kornilova ES. 2003. C-Cbl - polyfunctional regulator of intracellular processes. Tsitologiya. 45 (7):714-721. Review. Melikova MS, Blagoveshchenskaia AD, Nikol'skii NN, Kornilova ES. . 2002. Effect of an vacuolar proton pump inhibitor bafilomycin A1 on the intracellular processing of markers for receptor-mediated and liquid phase endocytosis. Tsitologiya (Rus).;44 (8):807-16. Kharchenko MV, Aksenov ND, Kornilova ES. 2002. Effect of a hypertonic sucrose solution and 5-(N,N-hexamethylene)-amiloride on receptor-mediated and liquid-phase endocytosis.Tsitologiya; (Rus) 44(7):681-90 Mozhenok TP, Leont'eva EA, Beliaeva TN. 2002. Effects of cationic vectors complexed with plasmid DNA on the phagosome-lysosome fusion in murine peritoneal macrophages and J744 macrophages.Tsitologiya(Rus);44(9):825-9 Zheleznova NN, Melikova MS, Nikol'skii NN, Kornilova ES. 2001.The role of Src-kinase in the regulation of endocytosis of EGF-receptor complexes. I. Dynamics of EGF internalization, recycling, sorting, and degradation during inhibition of Src-kinase activity. Tsitologiya (Rus).;43(12):1136-45. Krolenko S.A., Lucy J.A. 2001. Reversible vacuolation of T-tubules in skeletal muscle: mechanisms and implications for cell biology. Int. Rev. Cytol. 202: 243-298. Zheleznova NN, Nikol'skii NN, Kornilova ES. 2001. Effect of wortmannin on endocytosis of epidermal growth factor receptors. Tsitologiya. (Rus).;43(2):156-65 Melikova M.S., Blagoveshchenskaya A.D., Nikolsky N.N., Kornilova E.S. 2001. Influence of vacuolar proton pump inhibitor Bafilomycin A1 on intracellular processing of receptor-mediated and fluid phase endocytosis markers. Mol. Biol. Cell. 12 344a-345a. Mozhenok TP, Beliaeva TN, Bulychev AG, Leont'eva EA. 2000. Effect of polyamine synthesis inhibitors separately and in combination with epidermal growth factor on fusion of lysosomes with phagosomes and F-actin level in mouse peritoneal macrophages. Tsitologiya. (Rus). 42(6):573-7. Avrov KO, Aksenov ND, Melikova MS, Nikol'skii NN, Kornilova ES. 1999. Endocytosis of EGF-receptor complexes at various cell cycle stages. Tsitologiya. (Rus). 41(12):1007-13 Sokolova IP, Arnautov AM, Blagoveshchenskaia AD, Nikol'skii NN, Kornilova ES. 1998. Effect of nocodazole on endocytosis of epidermal growth factor receptor. Tsitologiya. (Rus). ;40(10):855-61. Sokolova IP, Arnautov AM, Nikol'skii NN, Kornilova ES. 1998. Studies of small GTPase Rab7 association with endosomes of cells expressing normal and mutant forms of epidermal growth factor receptors. Tsitologiya. (Rus). 40(10):862-8. T. Mozhenok, T. Belyaeva, A .Bulychev, I .Kuznetsova, E .Leontieva, M.Faddejeva. 1998. Effects of some biologically active compounds on phagosome-lysosome fusion in peritoneal macrophages of mice. Cell Biol. Int. 22: 465-472. Gilbert A., Jadot M., Leontieva E., Wattiaux-De Coninc S., Wattiaux R. 1998. DeltaF508 CFTR localizes in the endoplasmic reticulum-Golgi intermediate compartment in cystic fibrosis cells. Exp.Cell Res. 242. : 144-152. S.A. Krolenko, W.B. Amos, S.C. Brown, M.V. Tarunina, J.A. Lucy. 1998. Accessibility of T-tubule vacuoles to extracellular dextran and DNA: mechanism and potential application of vacuolation. J. Muscle Res. and Cell Motil. V. 19: 603-611. Kornilova E., Sorkina T., Beguinot L., Sorkin A. 1996 Carboxyl-terminal receptor domain 1022-1123 is responsible for the lysosomal targeting of EGF receptors. J.B.C., 271: 30340-30346. Krolenko SA, Adamian SIa. 1996. The effect of membrane-transport inhibitors on the vacuolization of skeletal muscle fibers induced by glycerin removal from them. Tsitologiya. (Rus). 38(7):751-7. Bulychev AG, Mozhenok TP .1996 .The vacuolar apparatus and membrane fusion in the cell. Tsitologiya. (Rus).;38(10):1001-35. Review. Krolenko S.A., Amos W.B., Lucy J.A. 1995. Reversible vacuolation of the transverse tubules of frog skeletal muscle: a confocal fluorescence microscopy study. J. Muscle Res. Cell Motil. V.16: 401-411. Sokolova IP, Vdovina IB, Kornilova ES, Nikol'skii NN. 1995. The compartmentalization of the epidermal growth factor (EGF) during endocytosis in cells expressing a normal EGF receptor and one lacking the major autophosphorylation sites. Tsitologiya. (Rus).;37(9-10):873-82. Wels W., Beerli R., Hellman P., Schidt M., Marte B.M., Kornilova E.S., Hekele A., Mendelsohn J., Groner B., Hynes E. 1995. GF receptor and p185erb-2-specific single-chain antibody toxins differ in their cell-killing activity on tumor cells expressing both receptor proteins. Int. J. Cancer. 60: 137-144. E.S. Kornilova, D. Taverna, W. Hoeck and N.E.Hynes. 1992. Surface expression of erb B-2 protein is post-transcriptionally regulated in mammary epithelial cells by epidermal growth factor and by culture density. Oncogene, 7: 511-519. Sorkin A., E.Kornilova, S. Krolenko. 1992. Two recycling pathways of epidermal growth factor receptor complexes in A431 cells. NATO ASI Series, Vol H62: (180-186) Endocytosis. Ed. by P. J. Courtoy, Springer - Verlag, Berlin- Heidelberg. Sorkin A., Kornilova E., Teslenko L., Sorokin A., Nikolsky N. 1988. Recycling of epidermal growth factor-receptor complexes in A431 cells. BBA, 1011: 88-96. |