| Laboratory of Ionic Mechanisms of Cell Signaling |
Yuri A. NEGULYAEVD.Sci., Ph.D.phone: +7(812)297-14-97
List of publications in ResearcherID or in Google Scholar 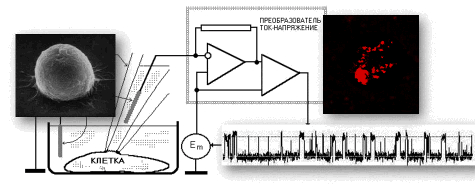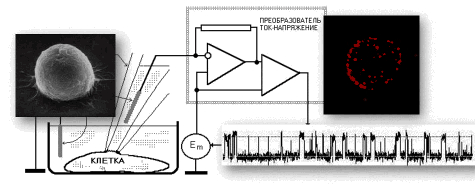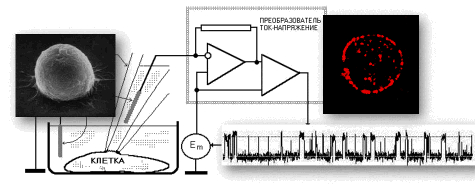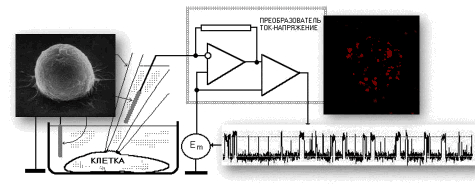 |

|

Elena A. Morachevskaya
|

Svetlana B. Semenova
|

Vladislav I. Chubinski-Nadezhdin
|

Anastasiya V. Sudarikova
| 
Irina O. Vassilieva
| 
Leonid S. Shuyskiy
|

Valeriya Yu.Vassilieva
|
|
Research Interests The main attention of the laboratory is focused on studying the cation-transporting channels in the plasma membrane of mammalian cells. We are also highly interested in the detection of physiologically significant pathways of activation and regulation of the various types of channels, whose functioning contributes to ion homeostasis and signal transduction of the cell, including formation of calcium response. The most important directions of studies are associated with evaluating importance of the submembrane cytoskeleton and membrane lipids in realization of the key functions of channel-forming proteins. Our experimental work is focused on identification of ion channels in living cells on basis of their biophysical characteristics. The obtained and published data of priority indicates functional coupling of cation channels with cortical actin network. It was shown, that activation and inactivation of non-voltage-gated sodium channels depend directly on the processes of disassembly and assembly of the submembrane microfilaments. The research into the possible participation of G-proteins in regulation of channels showed that F-actin rearrangement could be an inevitable link in the chain of signal transduction. For the recent years the work of the Laboratory has been based on the use of different functional approaches – first of all, on the estimation of activity and properties of single channels using electrophysiological recording of ion currents. The leading technique used in the investigations is the patch clamp. By now the variety of used approaches and methods has been significantly enlarged; first of all, imaging techniques and biochemical methods have been mastered, and current possibilities of the laboratory allow the functional characteristics of the channels to be supplemented by data concerning the channels' localization, molecular organization. Since 1996, the research of the Laboratory has been constantly supported by grants from the Russian Foundation for Basic Research, the Programs of the Presidium of the Russian Academy of Sciences "Molecular and Cell Biology" and the Russian Science Foundation. |
|
Selected Publications (1994-2017): * Sudarikova AV, Vasileva VY, Vassilieva IO, Negulyaev YA, Morachevskaya EA, Chubinskiy-Nadezhdin VI. Extracellular protease trypsin activates amiloride-insensitive sodium channels in human leukemia cells. J Cell Biochem. 2019 Jan;120(1):461-469. | PubMed | Sudarikova AV., Chubinskiy-Nadezhdin VI., Vasileva VY., Vassilieva IO., Morachevskaya EA, Negulyaev YA. [Novel activatory mechanism of actin-gated sodium channels in K562 cells]. Tsitologiya , 2018, 60(10), 821-825. [in russian], | free pdf in russian | Chubinskiy-Nadezhdin V.I., Efremova T.N., Negulyaev Y.A., Morachevskaya E.A. [Coupled activation of mechanosensitive and calcium-dependent potassium channels in 3T3 and 3T3-SV40 cells]. Tsitologiya., 2018, 60(1), pp. 14–20. [in russian], |free pdf in russian | Revittser A.V., Negulyaev Y.A. [Influence of atrial natriuretic peptide on migration of mesenchymal stem cells, gained from perirenal rat fat]. Tsitologiya.. 2018. 60 (12): 983 – 986. [in russian], | free pdf in russian | Revittser A.V., Negulyaev Y.A. [Adipogenic differentiation of human fetal bone mar-row derived mesenchymal stem cells using rosiglitazone]. Tsitologiya., 2018, 60(4), pp. 273–278. [in russian], | pdf in russian | Shuyskiy L.S., Levchenko V.V., Negulyaev Y.A. Staruschenko A.V., Ilatovskaya D.V. The role of the adapter protein MIM in the actin-dependent regulation of epithelial sodium channels (ENaC). ACTA NATURAE, 2018, 10(2), pp. 8-14. | pdf | Irina O. Vassilieva, Galina F. Reshetnikova, Alla N. Shatrova, Nataliya V. Tsupkina, Marianna V. Kharchenko, Larisa L. Alekseenko, Nikolay N. Nikolsky, Elena B. Burova. Senescence-messaging secretome factors trigger premature senescence in human endometrium-derived stem cells. Biochemical and Biophysical Research Communications, 2018, 496(4), pp. 1162-1168. | PubMed | Cherezova A.L., Negulyaev Y.A., Zenin V.V., Semenova S.B. [Extracellular pH regulates the calcium influx in Jurkat-cells]. Tsitologiya, 2017, 59(9), pp. 595-600. [in russian], |free pdf in russian | Chubinskiy-Nadezhdin VI, Vasileva VY, Pugovkina NA, Vassilieva IO, Morachevskaya EA, Nikolsky NN, Negulyaev YA. Local calcium signalling is mediated by mechanosensitive ion channels in mesenchymal stem cells. Biochemical and Biophysical Research Communications, 2017, 482(4):563-568. | PubMed | Semenova S.B. [TRP Channels in the endosomal pathway]. Tsitologiya, 2017, 59 (2), pp. 87–98. [in russian]. |free pdf in russian | Chubinskiy-Nadezhdin VI, Negulyaev YA, Morachevskaya EA. Simvastatin induced actin cytoskeleton disassembly in normal and transformed fibroblasts without affecting lipid raft integrity. Cell Biol Int., 2017, 41(9), pp. 1020-1029. | PubMed | Mamenko MV, Boukelmoune N, Tomilin VN, Zaika OL, Jensen VB, O'Neil RG, Pochynyuk OM. The renal TRPV4 channel is essential for adaptation to increased dietary potassium. Kidney Int., 2017, 91(6):1398-1409. | PubMed | Tomilin V.N., Cherezova A.L., Negulyaev Y.A., Semenova S.B. TRPV5/V6 Channels Mediate Ca2+ Influx in Jurkat T Cells Under the Control of Extracellular pH. J Cell Biochem., 2016, 117:197-206. | PubMed | Shatrova AN, Mityushova EV, Vassilieva IO, Aksenov ND, Zenin VV, Nikolsky NN, Marakhova II. Time-dependent regulation of IL-2R [alpha]-chain (CD25) expression by TCR signal strength and IL-2-induced STAT5 signaling in activated human blood T lymphocytes. PLoS One., 2016, Dec 9; 11(12):e0167215. | PubMed | Kukhtevich IV, Belousov KI, Bukatin AS, Chubinskiy-Nadezhdin VI, Vasileva VY, Negulyaev YA, Evstrapov AA Microfluidic Chips for the Study of Cell Migration under the Effect of Chemicals. Technical Physics Letters, 2016, Vol. 42, No. 5, pp. 478–481. Belousov KI, Bukatin AS, Chubinskiy-Nadezhdin VI, Vasilyeva VY, Negulyaev YA, Evstrapov AA, Kukhtevich IV. Microfluidic device with Y-shaped design for study of cell migration in concentration gradient of chemoattractants. Nauchnoe priborostroenie., 2016, Vol. 26. No. 1, pp. 3–10. Hoover R.S., Tomilin V., Hanson L., Pochynyuk O., and Ko B. PTH modulation of NCC activity regulates TRPV5 Ca2+ reabsorption. Am J Physiol Renal Physiol., 2016, 310(2):F144-51 | PubMed | Mistry A.C., Wynne B.M., Yu L, Tomilin V., Yue Q., Zhou Y., Al-Khalili O., Mallick R., Cai H., Alli A.A., Ko B., Mattheyses A., Bao H.-F., Pochynyuk O., Theilig F., Eaton D.C., Hoover R.S. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. The Biochemical journal, 2016, 473: 3237–3252. | PubMed | Zaika O., Palygin O., Tomilin V., Mamenko M., Staruschenko A., Pochynyuk O. Insulin and IGF-1 activate Kir4.1/5.1 channels in cortical collecting duct principal cells to control basolateral membrane voltage. American Journal of Physiology-Renal Physiology. 2016, 310: F311–F321. | PubMed | Tomilin V, Mamenko M, Zaika O, Pochynyuk O. Role of renal TRP channels in physiology and pathology. Seminars in Immunopathology, 2016, 38:371–383. | PubMed | Mamenko M, Dhande I, Tomilin V, Zaika O, Boukelmoune N, Zhu Y, Gonzalez-Garay ML, Pochynyuk O, Doris PA. Defective Store-Operated Calcium Entry Causes Partial Nephrogenic Diabetes Insipidus. J Am Soc Nephrol. 27: 2035–2048, 2016. | PubMed | Sudarikova A.V., Tsaplina O.A., Chubinskiy-Nadezhdin V.I., Morachevskaya E.A., Negulyaev Y.A. Amiloride-insensitive sodium channels are directly regulated by actin cytoskeleton dynamics in human lymphoma cells. Biochemical and Biophisical Research Communication, 2015. 461(1):54-58. | PubMed | Ilatovskaya D.V., Levchenko V., Lowing A., Shuyskiy L. S., Palygin O., Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II - dependent activation of TRPC channels. Scientific Reports., 2015, doi: 10.1038/srep17637. | PubMed | Petrov Y.P., Negulyaev Y.A., , Tsupkina N.V. [Spreading of NCTC clone 929 cells after reseeding]. Tsitologiya. 2015. 57(5):370–378 [in russian]. | PubMed | Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, Staruschenko A. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney International, 2014, 86(3):506-514. | PubMed | Chubinskiy-Nadezhdin VI, Negulyaev YA, Morachevskaya EA. Functional coupling of ion channels in cellular mechanotransduction. Biochemical and Biophisical Research Communication, 2014. 451(3):421–424. | PubMed | Petrov Iu.P., Neguliaev Iu.A., , Tsupkina N.V. [Response of HeLa cells to mitomycine С III. The analysis of nucleoli of mother and daughter cells] Tsitologiya, 2014, 56(2): 105–109 [in russian]. | PubMed | Lyublinskaya OG, Zenin VV, Shatrova AN, Aksenov ND, Zemelko VI, Domnina AP, Litanyuk AP, Burova EB, Gubarev SS, Negulyaev YA, Nikolsky NN. Intracellular oxidation of hydroethidine: Compartmentalization and cytotoxicity of oxidation products. Free Radical Biology and Medicine. 2014. 75:60-68. | PubMed | Petrov Iu.P., Neguliaev Iu.A., , Tsupkina N.V. [Colocalization of nuckleoli in cell nuclei of HeLa line] Tsitologiya, 2014, 56(3): 197–203 [in russian]. | PubMed | Pozdnyakov I., Matantseva O., Negulyaev Y., Skarlato S. Obtaining Spheroplasts of Armored Dinoflagellates and First Single-Channel Recordings of Their Ion Channels Using Patch-Clamping. Marine drugs. 2014. 12(9):4743-4755. | PubMed | Petrov Iu.P., Neguliaev Iu.A., , Tsupkina N.V. [Morphology of NCTC cells upon a contact with type I collagen added to culture medium] Tsitologiya. 2014. 56(8):591–598 [in russian]. | PubMed | Ilatovskaya DV, Chubinskiy-Nadezhdin V, Shuyskiy LS, Tomilin V, Pavlov TS, Palygin O, Staruschenko A and Negulyaev YA. Arp2/3 complex inhibitors adversely affect actin cytoskeleton remodeling in the cultured murine kidney collecting duct M-1 cells. Cell and Tissue Research, 2013, Sep 15, DOI 10.1007/s00441-013-1710-y. | PubMed | Chubinskiy-Nadezhdin VI, Efremova TN, Khaitlina SY and Morachevskaya EA. Functional impact of cholesterol sequestration on actin cytoskeleton in normal and transformed fibroblasts. Cell Biol. Int. 2013; 37: 617–623. | PubMed | Ilatovskaya DV, Staruschenko A. Single channel analysis of TRPC channels in the podocytes of the freshly isolated glomeruli. From: Methods in Molecular Biology. Ion Channels: Methods and Protocols. 2013, Humana Press Inc. 998:355-69, PMID: 23529444. | PubMed | Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol. 2013 Jan 1; 304 (1): 102–111. | PubMed | Chubinskiy-Nadezhdin VI, Sudarikova AV, Nikolsky NN, Morachevskaya EA. Role of submembranous actin cytoskeleton in regulation of non-voltage-gated sodium channels. Dokl Biochem Biophys. 2013 May-Jun;450:126-9. doi: 10.1134/S1607672913030010. | PubMed | Tomilin VN, Vassilieva IO, Marakhova II, Negulyaev YA, Semenova SB. The functional characteristics of TRPV5 and TRPV6 channels in normal and transformed human blood lymphocytes Tsitologiya, 2013, 55 (5): 300–306 [in russian]. |free pdf in russian | Petrov IuP, Neguliaev IuA, , Tsupkina NV. [Response of HeLa cells to Mitomycine C. II. Morphometry of the cells.] Tsitologiya, 2013, 55(12): 879–885 [in russian]. | PubMed | Vassilieva IO, Tomilin VN, Marakhova II, Shatrova AN, Negulyaev YA, Semenova SB. Expression of Transient Receptor Potential Vanilloid Channels TRPV5 and TRPV6 in Human Blood Lymphocytes and Jurkat Leukemia T Cells. The Journal of Membrane Biology. 2013, 246 (2): 131–140. | PubMed | Sudarikova AV, Vassilieva IO, Morachevskaya EA, Neguliaev IuA. [Molecular and functional identification of sodium channels in K562 cells]. Tsitologiya. 2012, 54 (7): 573–579 [in russian]. | PubMed | Ilatovskaya DV, Pavlov TS, Negulyaev YA, Staruschenko Av. [Regulation of TRPC6 Channels by NonSteroid AntiInflammatory Drugs]. Biologicheskie membrany. 2012, 29(3), 200–208 [in russian]. Efremova TN, Chubinskiy-Nadezhdin VI, Khaitlina SY, Morachevskaya EA. [Assembly of actin filaments induced by sequestration of membrane cholesterol in transformed cells]. Tsitologiya. 2012, 54(6): 508–514 [in russian]. | PubMed | Petrov IuP, Neguliaev IuA, , Tsupkina NV. [Analysis of the cell cycle duration of cells of permanent line L-929].Tsitologiya. 2012, 54(3): 214–217 [in russian]. | PubMed | Petrov IuP, Neguliaev IuA, , Tsupkina NV. [Dynamics of spreading of cells of L-929 line after the mitosis].Tsitologiya. 2012, 54(4): 307–312 [in russian]. | PubMed | Petrov IuP, Neguliaev IuA, , Tsupkina NV. [The estimation of similarity in characteristics of the post-mitotic daughter L-929 cells during their migration along the substrate]. Tsitologiya. 2012, 54(5): 405–411 [in russian]. | PubMed | Mamenko M., Zaika O., Ilatovskaya DV, Staruschenko A. and Pochynyuk O. Angiotensin II increases activity of the Epithelial Na+ Channel (ENaC) in the distal nephron additively to aldosterone. J Biol Chem. 2012, 287(1): 660-671. | PubMed | Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko AV. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J. 2011, 25(8), 2688-99. | PubMed | Chubinskiy-Nadezhdin VI, Negulyaev YA, Morachevskaya EA. Cholesterol depletion-induced inhibition of stretch-activated channels is mediated via actin rearrangement. Biochem Biophys Res Commun. 2011, V.412(1), 80-5. | PubMed | Ilatovskaya DV, Pavlov TS, Negulyaev YA, Staruschenko AV. [Mechanisms of epithelial sodium channel (ENaC) regulation by cortactin: involvement of dynamin]. Tsitologiya. 2011, 53 (11), 903-910 [in russian]. | PubMed | Petrov IuP, Neguliaev IuA, Tsupkina NV. [The position of cleavage furrow in cultivated cells on the example of lines L-929 and CHO]. Tsitologiya. 2011, 53 (11), 839-847 [in russian]. | PubMed | Petrov IuP, Neguliaev IuA. [The average cell size is a factor reflecting the interaction of the CHO cells during proliferation]. Tsitologiya. 2011, 53 (8), 671-678 [in russian]. | PubMed | Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC). Am J Physiol Renal Physiol. 2011, Jun 22. | PubMed | Ilatovskaya DV, Levchenko V, Ryan RP, Cowley AW Jr, Staruschenko A. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem Biophys Res Commun. 2011, V.57(5), 996-1002. | PubMed | Karpushev AV, Levchenko V, Ilatovskaya DV, Pavlov TS, Staruschenko AV. Novel role of Rac1/WAVE signaling mechanism in regulation of the epithelial Na+ channel. Hypertension. 2011, V.57(5), 996-1002. | PubMed | Karpushev AV, Ilatovskaya DV, Pavlov TS, Negulyaev YA Staruschenko AV. Intact cytoskeleton is required for small G protein dependent activation of the epithelial Na+ channel. PLoS One. 2010, V.5 (1), e8827. | PubMed | Filatova N.A., Chubinskij-Nadezhdin V.I., Ivanov V.A., Morachevskaia E.A. [Sensitivity to attack of natural killers depends on integrity of lipid rafts in plasma memebrane of transformed cells]. Tsitologiya. 2010, 52 (12), 983-989 [in russian]. | PubMed | free pdf in russian | Karpushev AV, Ilatovskaya DV, and Staruschenko AV. The actin cytoskeleton and small G protein RhoA are not involved in flow-dependent activation of ENaC. BMC Res Notes. 2010, 3, 210. | PubMed | Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol. 2010 May, 21 (5), 833-43. | PubMed | Karitskaya I, Aksenov N, Vassilieva I, Zenin V, Marakhova I. Long-term regulation of Na,K-ATPase pump during T-cell proliferation. Pflugers Arch. 2010, Sep; 460 (4), 777-89. | PubMed | Semenova SB, Vassilieva IO, Fomina AF, Runov AL, Negulyaev YA . Endogenous expression of TRPV5 and TRPV6 calcium channels in human leukemia K562 cells. Am J Physiol Cell Physiol. 2009, 296 (5), 1098-104. | PubMed | Sviderskaya EV, Easty DJ, Lawrence MA, Sanchez DP, Negulyaev YA, Patel RH, Anand P, Korchev YE, Bennett DC. Functional neurons and melanocytes induced from immortal lines of postnatal neural crest-like stem cells. FASEB J. 2009, 23 (9), 3179-92. | PubMed | Sudarikova A.V., Chubinskij-Nadezhdin V.I., Neguliaev Iu.A., Morachevskaia E.A. [Functional properties of sodium channels in cholesterol-depleted K562 cells] Tsitologiya. 2009, 51 (8), 676-83 [in russian]. | PubMed | free pdf in russian | Vachugova(Ilatovskaya) DV, Morachevskaya EA. [Mechanosensitivity of cationic channels of DEG/ENAC family] Tsitologiya. 2009, 51 (10), 806-814 [in russian]. | PubMed | free pdf in russian | Julia Gorelik, Nadire N. Ali, Siti H. Sheikh Abdul Kadir, Max Lab, Petra Stojkovic, Lyle Armstrong, Elena V. Sviderskaya, Yuri A. Negulyaev, David Klenerman, Dorothy C. Bennett, Majlinda Lako, Sian E. Harding, Miodrag Stojkovic, and Yuri E. Korchev. Non-invasive Imaging of Stem Cells by Scanning Ion Conductance Microscopy: Future Perspective. TISSUE ENGINEERING: Part C. 2008, 14 (4), 311-318. | PubMed | Vasil'eva IO, Neguliaev IuA, Marakhova II, Semenova SB. [TRPV5 and TRPV6 calcium channels in human T cells] Tsitologiya. 2008, 50 (11), 953-957 [in russian]. | PubMed | free pdf in russian | Morachevskaya EA, Sudarikova AV, Negulyaev YA. Mechanosensitive channel activity and F-actin organization in cholesterol-depleted human leukaemia cells. Cell Biology International. 2007, 31, 374-381. | PubMed | Semenova SB, Negulyaev YA. [The endogenous cation-transporting channels in human myeloid leukemia cells.] BIOLOGICHESKIE MEMBRANY. 2006, 23 (4), 321-329 [in russian]. Staruschenko AV, Sudarikova AV, Negulyaev YA, Morachevskaya EA. Magnesium permeation through mechanosensitive channels: single-current measurements. Cell Research. 2006, 16 (8), 723-730. | PubMed | Semenova SB, Fomina AF, Vassilieva IO, Negulyaev YA. Properties of Mg2+-dependent cation channels in human leukemia K562 cells. Journal of Cellular Physiology. 2005, 205 (3), 372-378. | PubMed | Staruschenko AV, Negulyaev YA, Morachevskaya EA. Actin cytoskeleton disassembly affects conductive properties of stretch-activated cation channels in leukaemia cells. Biochimica et biophysica acta. 2005, 1669 (1), 53-60. | PubMed | Shumilina EV, Khaitlina SY, Morachevskaya EA, Negulyaev YA. Non-hydrolyzable analog of GTP induces activity of Na+ channels via disassembly of cortical actin cytoskeleton. FEBS Letters. 2003 July 17, 547 (1-3), 27-31. | PubMed | Shumilina EV, Negulyaev YA, Morachevskaya EA, Hinssen H, Khaitlina SY. Regulation of sodium channel activity by capping of actin filaments. Molecular Biology of the Cell. 2003, 14 (4), 1709-1716. | PubMed | Staruschenko AV, Vedernikova EA. (Morachevskaya EA). Mechanosensitive cation channels in human leukaemia cells: calcium permeation and blocking effect. Journal of Physiology. 2002, 541, 81-90. | PubMed | Sergeev IaS, Glushankova LN, Neguliaev IuA. [Store-operated cationic channels in human myeloid leukaemia K562 cells]. Tsitologiya. 2002, 44 (6), 545-550 [in russian]. | PubMed | Starushchenko AV, Neguliaev IuA, Morachevskaia EA.[Inhibiting and stimulating effect of amiloride on potential-dependent cation channels in K562 cells]. Tsitologiya. 2002, 44 (7), 676-680 [in russian]. | PubMed | A.V. Staruschenko, Y.A. Negulyaev, E.A. Vedernikova (E.A. Morachevskaya) Stretch-activated ion channels in human leukemia cells. Neurophysiology. 2000, 32 (3), 180-181. Negulyaev YA, Khaitlina SY, Hinssen H, Shumilina EV, Vedernikova EA. (Morachevskaya EA.) Sodium channel activity in leukemia cells is directly controlled by actin polymerization. The Journal of Biological Chemistry. 2000, 275 (52), 40933-7. | PubMed | Korchev YE, Negulyaev YA, Edwards CR, Vodyanoy I, Lab MJ. Functional localization of single active ion channels on the surface of a living cell. Nature Cell Biology. 2000, 2 (9), 616-619. | PubMed | Negulyaev YA, Markwardt F. Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neuroscience Letters. 2000, 279 (3), 165-168. | PubMed | Starushchenko AV, Mamin AG, Neguliaev IuA, Vedernikova EA.(Morachevskaya EA.). [Activation of mechanosensitive ion channels in plasma membrane of K562 cells]. Tsitologiya. 2000, 42 (7), 669-674 [in russian]. | PubMed | Vedernikova EA (Morachevskaya EA.), Maksimov AV, Neguliaev IuA. [Functional characteristic and molecular topology of voltage independent sodium channels] Tsitologiya. 1999, 41 (8), 658-66. Review [in russian]. | PubMed | Kiselyov K.I., Mamin A.G., Semyonova S.B., Mozhayeva G.N. Miniature Ca2+ channels in excised plasma-membrane patches: activation by IP-3. European Journal of Physiology (Pflugers Archiv). 1999, 437, 305-314. | PubMed | Semyonova S.B., Kiselyov K.I., Mozhayeva G.N. Low-conductivity calcium channels in the macrophage plasma membrane: activation by inositol-1,4,5-triphosphate. Neurosci Behav Physiol. 1999, 29 (3), 339-45. | PubMed | Semenova SB., Kiselev KI, Mozhaeva GN. [Low conductivity calcium channels in the plasmatic membrane of macrophages: activation with inositol 1,4,5-triphosphate]. Ross Fiziol Zh Im I M Sechenova. 1998, 84, 417-425 [in russian]. | PubMed | Maximov AV, Vedernikova EA (Morachevskaya EA), Hinssen H, Khaitlina SY, Negulyaev YA. Ca-dependent regulation of Na+-selective channels via actin cytoskeleton modification in leukemia cells. FEBS Lett. 1997, Jul 21, 412 (1), 94-96. | PubMed | Kiselyov KI, Mamin AG, Semyonova SB, Mozhayeva GN. Low-conductance high selective inositol (1,4,5)-trisphosphate activated Ca2+ channels in plasma membrane of A431 carcinoma cells. FEBS Lett. 1997, 407 (3), 309-12. | PubMed | Negulyaev, Y.A., Maximov A.V., Vedernikova E.A. (Morachevskaya EA), and Katina I.E.. Voltage-insensitive Na+ channels of different selectivity in human leukemic cells. General Physiology and Biophysics. 1997, 16, 163-173. | PubMed | Vedernikova EA (Morachevskaya EA.), Maksimov AV, Neguliaev IuA. [Functional properties and cytoskeletal-dependent regulation of sodium channels in leukemia cell membranes] Tsitologiia. 1997, 39, N12, 1142-1151. | PubMed | Kiselev KI, Mamin AG, Semenova SB, Mozhaeva GN. [A new type of IP3-sensitive highly selective calcium channels of low conductance in the plasma membrane of carcinoma A 431 cells.] Tsitologiia. 1997, 39 (6), 395-408 [in russian]. | PubMed | Negulyaev YA, Vedernikova EA (Morachevskaya EA), Maximov AV. Disruption of actin filaments increases the activity of sodium-conducting channels in human myeloid leukemia cells. Molecular Biology of the Cell. 1996, 7 (12), 1857-64. | PubMed | Negulyaev, Y.A., Vedernikova E.A. (Morachevskaya EA), Kinev A.V., and Voronin A.P. Exogenous heat shock protein hsp70 activates potassium channels in U937 cells. Biochimica et Biophysica Acta. 1996, 1282, 156-162. | PubMed | Barski, I.Y., Khomenkova, S.A., Negulyaev Y.A., Biktashev, A.G. Two-wave microfluorimeter for determining the concentration of intracellular calcium. Journal of Optical Technology. 1996, 63 (1), 66-68. Neguliaev IuA, Vedernikova EA, (Morachevskaya EA), Maksimov AV. [Aldosterone increases the activity level of Na+-conducting channels in chronic myeloid leukemia K562 cells] Dokl Akad Nauk. 1996 Aug, 349 (5), 701-703 [in russian]. | PubMed | Negulyaev YuA, Vedernikova EA (Morachevskaya EA), Mozhayeva GN. Several types of sodium-conducting channel in human carcinoma A-431 cells. Biochimica et Biophysica Acta. 1994, 1194 (1), 171-175. | PubMed | Negulyaev YA, Vedernikova EA. (Morachevskaya EA). Sodium-selective channels in membranes of rat macrophages. The Journal of Membrane Biology. 1994, 138 (1), 37-45. | PubMed | *Surnames of the authors worked at this time in laboratory are highlighted |
|
International collaboration
|